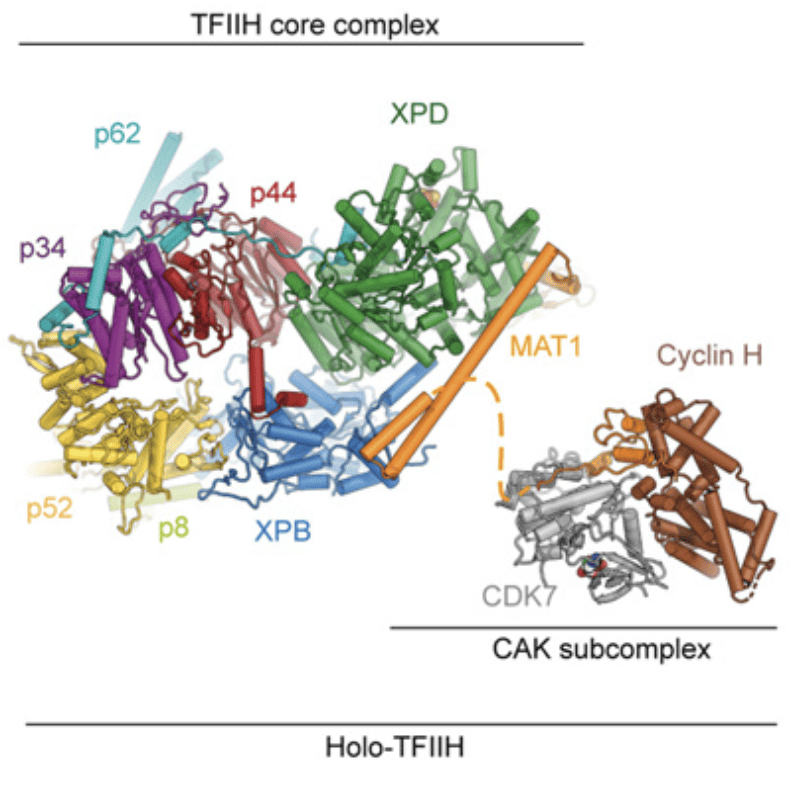
TFIIH is a large molecular complex that is essential for transcription initiation by RNA Pol II and the Nucleotide Excision DNA Repair (NER) pathway. Structural studies in our lab initially unraveled the molecular architecture of the TFIIH core complex, showing how the two ATP-consuming subunits, XPD and XPB are integrated and regulated by other structural elements in the complex. The structure reveals how the recruitment of XPB by p52 depends on a pseudo-symmetric dimer of homologous domains in these two proteins. With respect to XPD, the model shows that it is wrapped by numerous interactions with XPB, p62, p44, and MAT1, indicating how its activity can be tightly controlled and de-repressed only when its enzymatic function is needed in NER to scan for the damaged DNA.
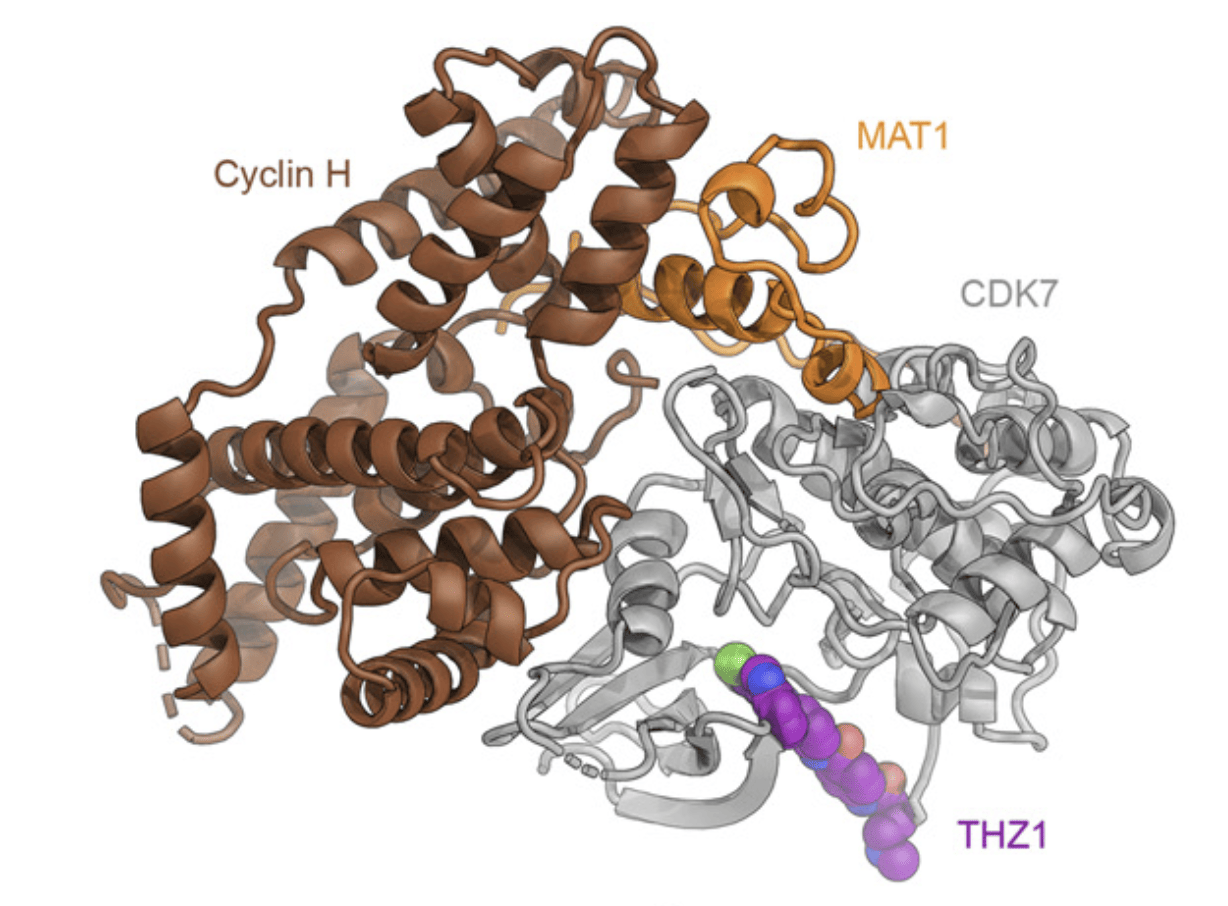
However, the CDK-activating kinase (CAK) module of TFIIH remained refractive to structure determination due to its flexible tethering to the core-TFIIH. The CAK impinges on two key regulatory networks that ensure appropriate cell growth and timely cell division: control of gene expression and cell cycle regulation. Recently, we were able to reconstitute the CAK and solve its structure to 2.8 Å resolution, revealing its architecture and the interactions between its regulatory elements. Additionally, we have obtained the structure of the CAK in complex with a small-molecule inhibitor that is being tested as an anti-cancer drug. The three proteins that form the CAK are the CDK-Cyclin pair CDK7-Cyclin H, and MAT1, which serves both architectural and regulatory functions. Our studies indicate that MAT1 not only integrates the CAK module to the TFIIH core, but also stabilizes the subcomplex and keeps CDK7 in an active conformation that is primed for phosphorylating the C-terminal domain of RPB1 in RNA Pol II to trigger the start of the elongation phase of transcription. Furthermore, the reconstructed volume corresponds to a mass of ~84 KDa, which is among the smallest asymmetric particles for which better than 3 Å resolution have been obtained.
- Greber, B. J. et al. The cryoelectron microscopy structure of the human CDK-activating kinase. Proc National Acad Sci 202009627 (2020) doi:10.1073/pnas.2009627117.
- J., G., B., B., T., D., J., F. & E., N. The complete structure of the human TFIIH core complex. Elife 8, e44771 (2019).
- Greber, B. J. et al. The cryo-electron microscopy structure of human transcription factor IIH. Nature 549, 414–417 (2017).
