In collaboration with the Kerfeld Lab we work on phycobilisomes (PBS): The PBS is an elaborate antenna that is responsible for light-harvesting in most cyanobacteria, the most abundant primary producers on Earth. The PBS captures incident sunlight and transfers the energy via a network of pigment molecules to the photosynthetic reaction centers. The PBS of the model organism Synechocystis sp. PCC 6803 consists of a core to which six rods are attached.
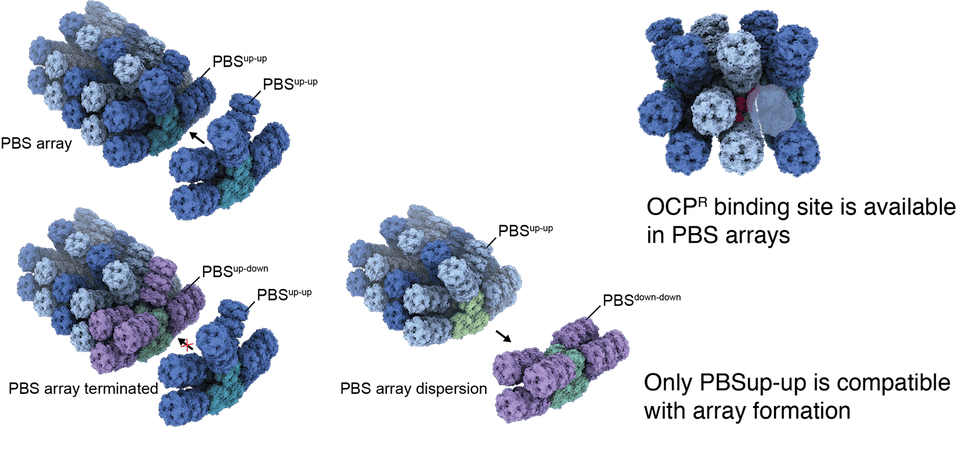
In the cell, PBSs are often arranged in arrays which efficiently pack light harvesting pigments on the surface of the thylakoid membrane and thus increase the overall photon absorption cross-section of a cell.
When the PBS absorbs excess light-energy, cyanobacteria trigger non-photochemical quenching: blue-green light converts the water-soluble, carotenoid containing photoreceptor Orange Carotenoid Protein (OCP) into the quenching red form OCPR that binds to the PBS antenna.
We solved the structure of the PBS and PBS-OCP using cryo-EM yielding resolutions ranging from 2.1 to 3.5Å. To overcome preferred particle orientations and protein instability we used streptavidin affinity grids made in-house.
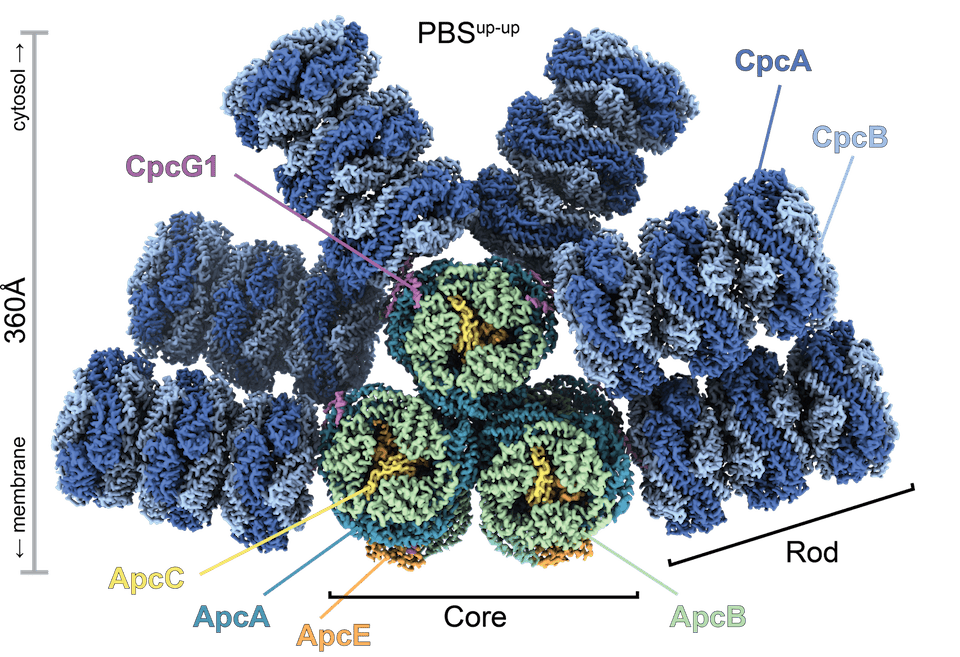
We identified three different unquenched PBS states in which two of the upper rods toggle between an ‘up’ and a ‘down’ conformation. To switch between ‘up’ and ‘down’, the linker protein CpcG1 undergoes a conformational change. The hinge region can adopt an extended and a compact form. Switching from one to the other leads to the movement of the rod. The rods move up/down by 62° and rotate around their long axis by 135°. Only the mobile rods are free to move, the static rods sterically block each other.
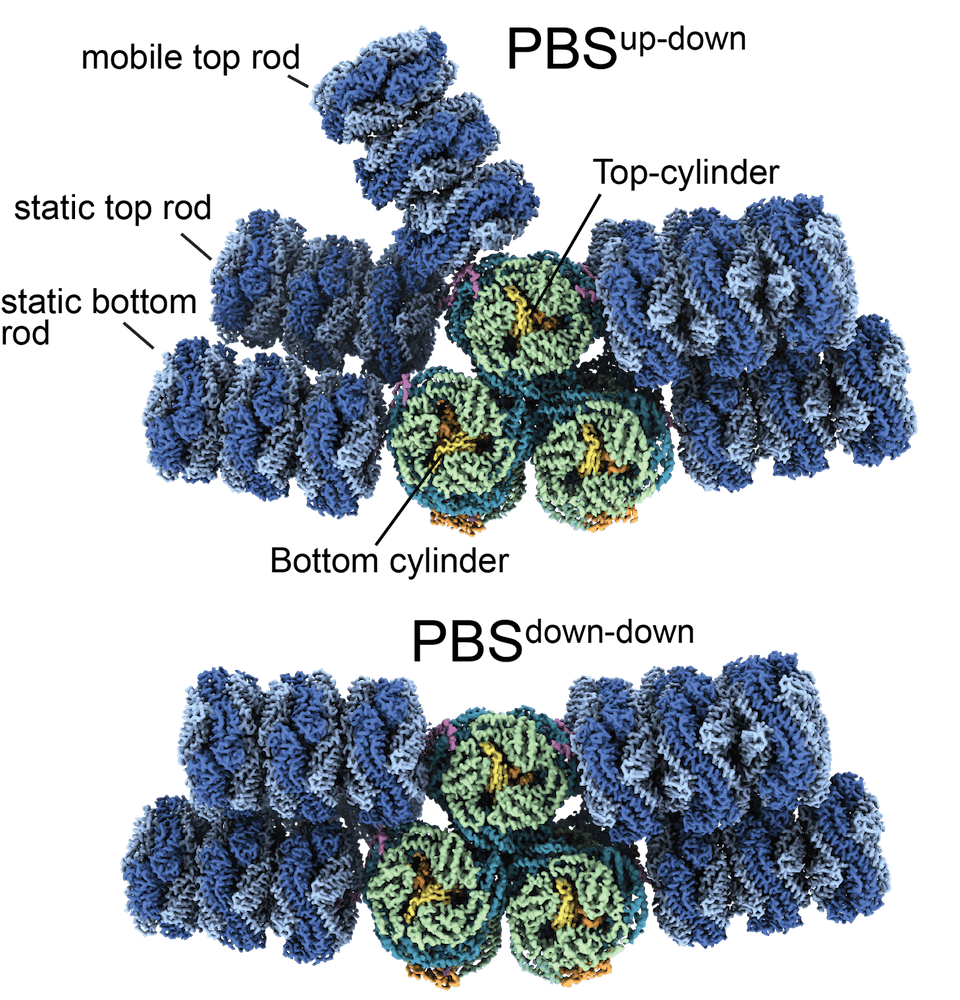
Computational modeling demonstrates that the different rod conformations do not affect light transfer efficiency. All forms transfer excitation from the rods to the bottom of the core within a few picoseconds.
The structure of the quenched PBS-OCPR complex reveals that contrary to previous expectations OCP binds away from the membrane, clasping on laterally to the core of the PBS. Only PBSup-up can accommodate OCPR as the binding site is blocked by the rod otherwise.
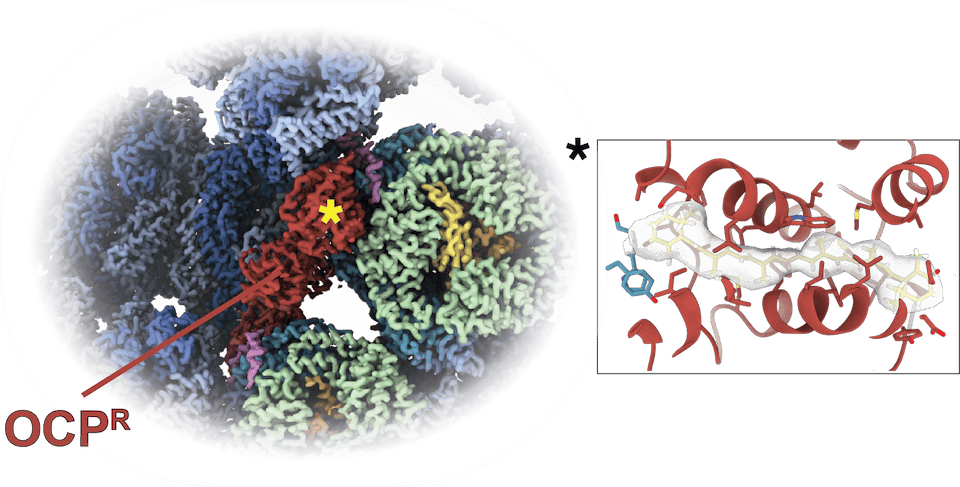
The carotenoid molecule within the quencher OCPR sits entirely within OCP’s N-terminal domain, unlike in the inactive precursor. It does not insert itself into the PBS. OCPR dimerizes on the surface of the PBS via its C-terminal domain.
One such dimer binds on each side of the PBS (4 OCPR total). OCPR is an efficient quencher, however only if 4 OCPR proteins are bound. Here too, computational modeling recapitulates the energy transfer and population life-times and the results are in agreement with experimental data.
We speculate that the different rod conformations regulate PBS array formation and OCP binding. PBSup-up can form arrays which can be quenched quickly with OCP if necessary. PBSdown-down cannot be quenched but also cannot form arrays. Such a system offers maximum flexibility in changing light conditions while maintaining a base supply with light
Our work helps to understand oxygenic photosynthesis which is an ancient cyanobacterial innovation that allowed complex life to emerge. It further contributes to establishing cyanobacteria as a platform for green biotechnologies. Normally, 60-80% of captured light energy is lost to non-photochemical quenching and we hope that our structures help to bioengineer photosynthesis in a useful way.
Structures of the Cyanobacterial Phycobilisome
Paul V. Sauer, Maria Agustina Dominguez-Martin, Henning Kirst, Markus Sutter, DavidBina, Basil J. Greber, Eva Nogales, Tomáš Polívka, Cheryl A. Kerfeld
bioRxiv 2021.11.15.468712; doi: https://doi.org/10.1101/2021.11.15.468712
Structure of the Quenched Cyanobacterial OCP-Phycobilisome Complex
Maria Agustina Dominguez-Martin, Paul V. Sauer, Markus Sutter, Henning Kirst, DavidBina, Basil J. Greber, Eva Nogales, Tomáš Polívka, Cheryl A. Kerfeld
bioRxiv 2021.11.15.468719; doi: https://doi.org/10.1101/2021.11.15.468719
