Polycomb Repressive Complex 2 (PRC2) trimethylates histone H3K27 to silence specific genes. It’s role is especially critical during development and for the maintenance of cell identity. In spite of its biological importance, when we began working on PRC2 little was known about the complex’s architecture and subunit organization.
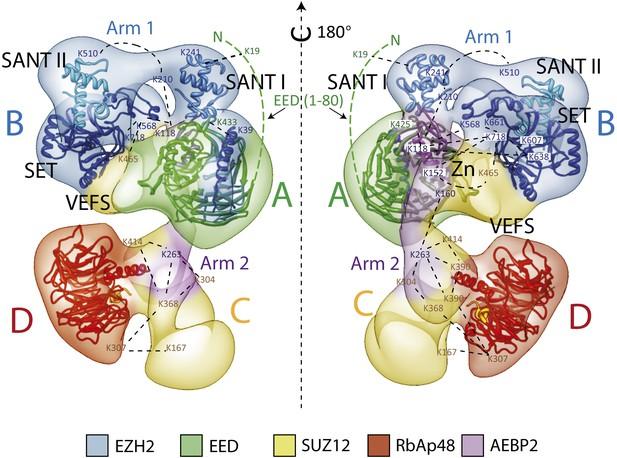
Prior to the resolution revolution, we reconstituted a tetrameric human PRC2 complex (Ezh2/EED/Suz12/RbAp48) with its cofactor AEBP2 and obtained the only available structural description of the complex (20 Å resolution) by negative stain. We used a tagging strategy to position all functional domains within the complex that showed that the Ezh2’s SET domain forms a core with the two activity-controlling elements, the WD40 domain of EED and the VEFS domain of Suz12.
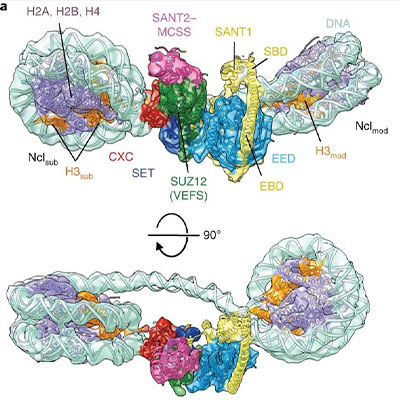
Building upon this foundational work, we used cryo-EM to solve structures of PRC2 bound to dinucleosome substrates revealing the structural basis for PRC2 stimulation by its own catalytic product that ultimately leads to spreading of the silencing mark. These structures show that PRC2 engages the nucleosomal DNA and is positioned to interact with the H3 tail of one nucleosome in the active site and an adjacent nucleosome through the allosteric site which explained previous biochemical experiments. Unfortunately, the bottom half of the PRC2 complex was absent from our reconstructions due to the tendency for PRC2 to fall apart at the air-water interface.
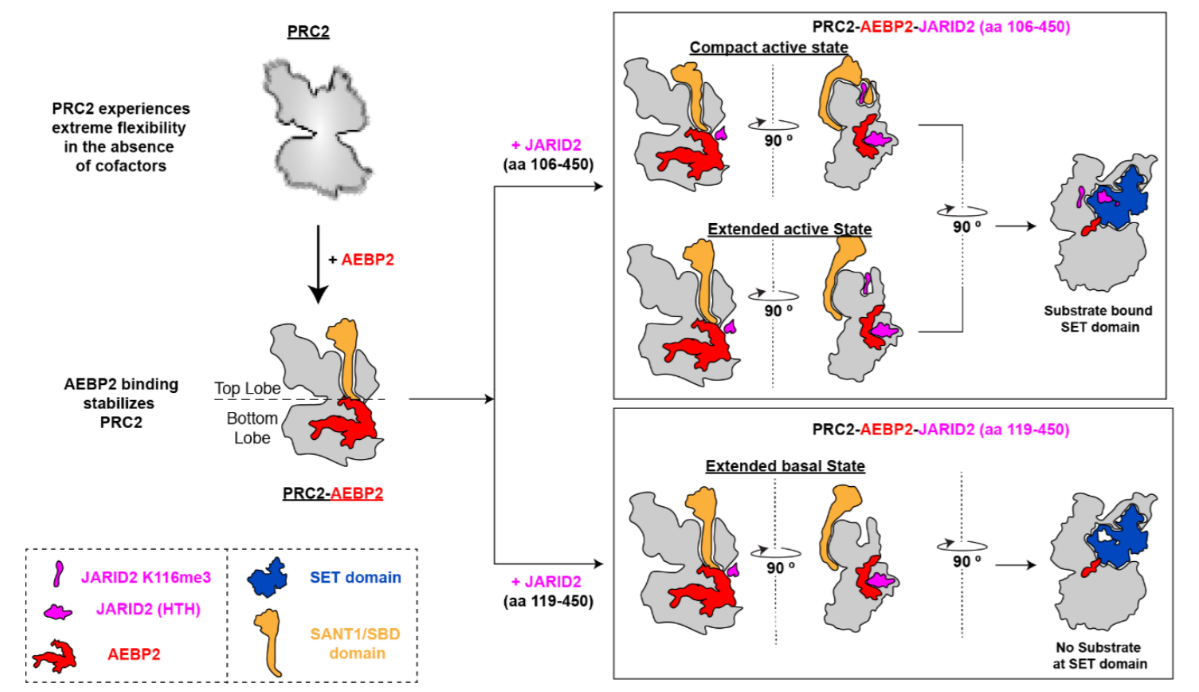
Additionally, we have characterized the first high resolution and first intact cryo-EM structure of the entire PRC2 complex with its cofactors AEBP2 and JARID2. The JARID2 cofactor, methylated by PRC2, mimics the H3 tail and is located in both the allosteric and active sites of PRC2 in our reconstructions. This work also captured two distinct conformations that we term the “extended active” and the “compact active” states that beautifully demonstrate the mechanism for allosteric stimulation through the binding of methylated peptides in the allosteric site.
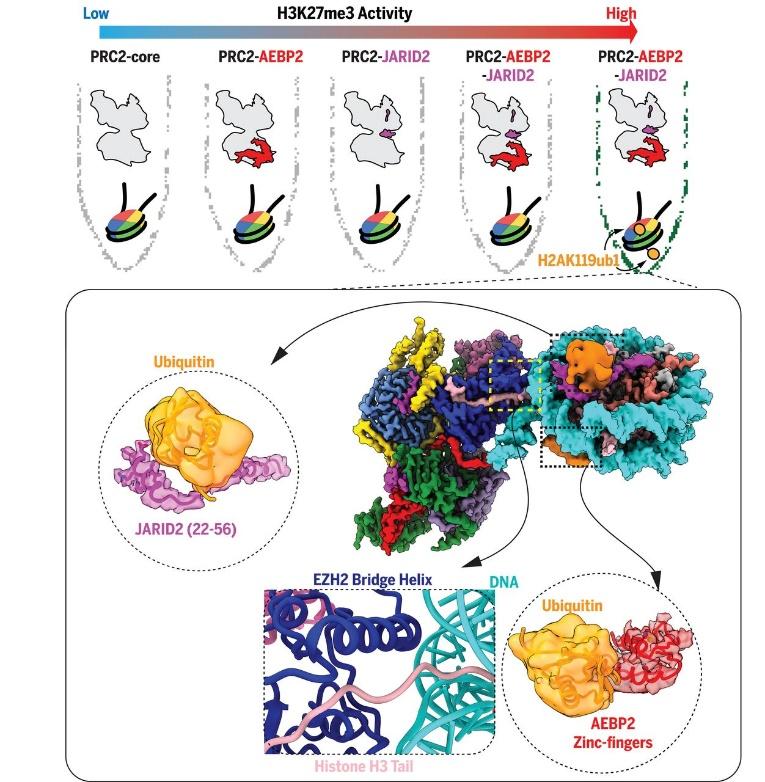
Most recently, we determined the first high resolution structure of an intact PRC2 complex bound to chromatin by using specialized streptavidin affinity grids which overcame the need for crosslinking and protected PRC2 from the air-water interface. This work detailed the interaction between PRC2 and the linker DNA of the nucleosome and identified an ordered “bridge helix” that interacts extensively with the nucleosomal DNA, includes residues that are automethylated by EZH2, and were previously predicted to be disordered. We could also, for the first time, observe the stabilizing interactions that guide the H3 tail into the EZH2 active site. Additionally, we used a H2AK119Ub modified nucleosome substrate to explore the role of PRC1-mediated crosstalk in PRC2 recruitment and activity. We found that the JARID2 and AEBP2 cofactors make extensive interactions with the nucleosome through H2AK119ub1 and H2A-H2B surfaces and our biochemical assays suggest there is increased activation of PRC2 in the presence of the ubiquitin moiety. Most importantly, this work supports critical roles of PRC2 cofactors in the regulation of PRC2 activity and recruitment that we are currently further exploring.
Relevant publications:
Vignesh Kasinath, Curtis Beck, Paul Sauer, Simon Poepsel, Jennifer Kosmatka, Marco Faini, Daniel Toso, Ruedi Aebersold, Eva Nogales JARID2 and AEBP2 regulate PRC2 in the presence of H2AK119ub1 and other histone modifications Science DOI: 10.1126/science.abc3393
Kasinath V, Poepsel S, Nogales E Recent structural insights into PRC2 regulation and substrate binding Biochemistry DOI: 10.1021/acs.biochem.8b01064
Vignesh Kasinath, Marco Faini, Simon Poepsel, Dvir Reif, Xinyu Ashlee Feng, Goran Stjepanovic, Ruedi Aebersold, Eva Nogales Structures of human PRC2 with its cofactors AEBP2 and JARID2 Science DOI: 10.1126/science.aar5700
Simon Poepsel, Vignesh Kasinath & Eva Nogales Cryo-EM structures of PRC2 simultaneously engaged with two functionally distinct nucleosomes Nature Structural & Molecular Biology doi:10.1038/s41594-018-0023-y
C.S. Huang, E. Nogales, C. Ciferri Molecular Architecture of the Polycomb Repressive Complex 2 Polycomb Group Proteins doi: 10.1016/B978-0-12-809737-3.00008-8
Claudio Ciferri, Gabriel C. Lander, Alessio Maiolica, Franz Herzog, Ruedi Aebersold and Eva Nogales (2012) Molecular architecture of Polycomb Repressive Complex 2. eLife, 1:e00005 Oct 30 2012 Epub.
